
Stratatech, a Madison company developing regenerative tissue as an alternative to skin grafts in treating burns, has submitted a biologics license application to the Food and Drug Administration, the company said Tuesday.
The “rolling submission” of the application for StrataGraft, a tissue product using human cells derived from discarded foreskins after circumcision, started in April and is now complete.
The move “brings us one step closer to our goal of providing StrataGraft skin tissue as a new treatment option for patients in the United States with deep partial-thickness thermal burns, if approved,” Dr. Steven Romano, executive vice president and chief scientific officer at Mallinckrodt, said in a statement.
England-based Mallinckrodt bought Stratatech in 2016 for $76 million. Stratatech, started in 2000, is based on research from the UW-Madison lab of pathologist Lynn Allen-Hoffmann.
Stratatech, which has about 100 employees, is located at University Research Park on Madison’s West Side, where the company completed a $28 million addition last year. The additional space, which tripled the company’s footprint at 535 Science Drive from 13,000 square feet to 35,000 square feet, will provide capacity to manufacture StrataGraft for continuing clinical trials and, if approved, commercial use, company officials said.
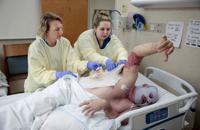
The goal for StrataGraft is to prevent or reduce the need to use painful autografts, or skin grafts from a patient’s own body, to cover up burn wounds and help them heal.
The FDA application is based on a study at 12 burn centers, in which 71 patients with deep second-degree burns received StrataGraft on one area of a burn injury and an autograft on a similarly burned site.
After three months, 83% of burn wounds treated with StrataGraft tissue were closed, compared to 86% of wounds treated with autografts, Mallinckrodt said.
About 4% of the area of StrataGraft-treated sites still needed autografts after three months, while 2% of the area treated by autografts required additional autografts at that stage. Overall, the use of StrataGraft resulted in a 98% reduction in wound areas needing autografts.
The 12 study sites included UW Hospital. Mallinckrodt has also conducted a study of StrataGraft in patients with third-degree burns and tested a related product, ExpressGraft, on patients with diabetic foot ulcers.
The FDA has designated StrataGraft an “orphan drug” and a “regenerative medicine advanced therapy,” which is expected to put it on a fast track for consideration.

Recent Comments