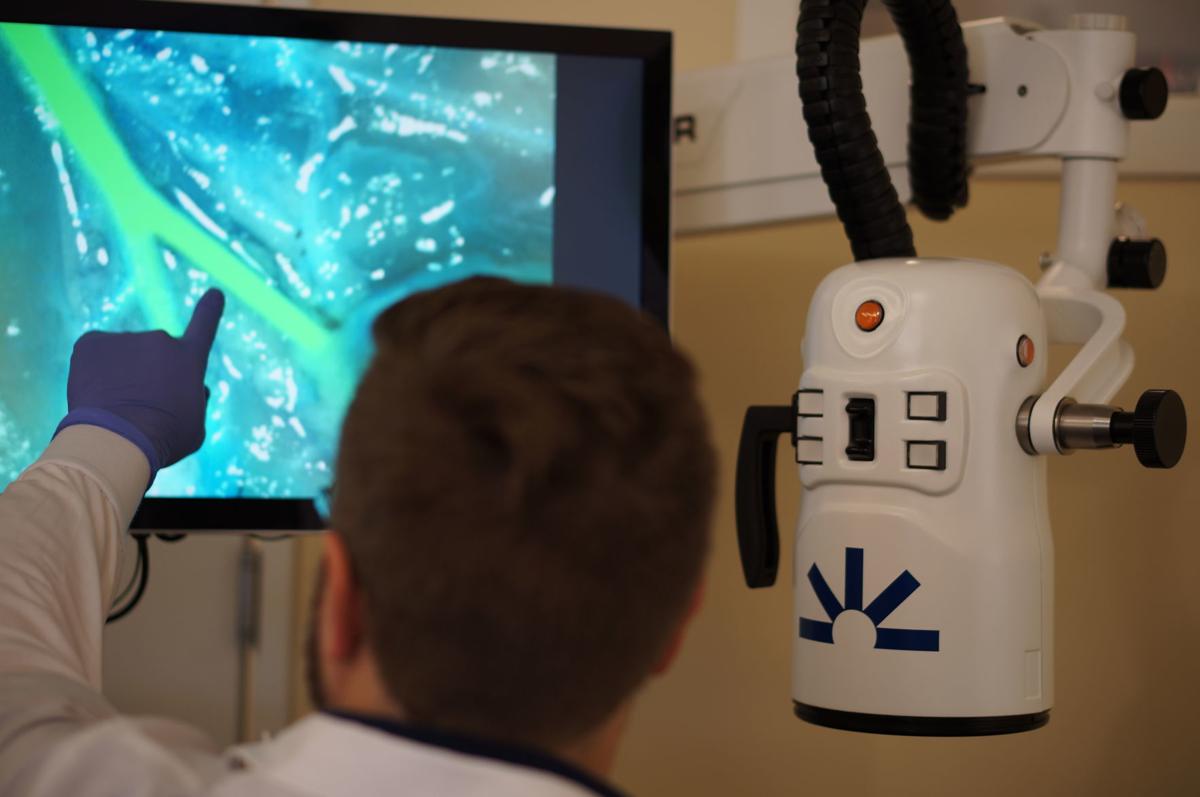
A device developed by a Madison company to help surgeons better see tissue during operations has been approved by the U.S. Food and Drug Administration.
OnLume’s fluorescence guided surgery system, approved Jan. 31, features a customized camera at the end of a long, articulating arm. It is used with FDA-approved indocyanine green dye to display fluorescent images on a monitor.
“It helps color code the tissue in the operating room so the surgeon can differentiate what looks normal from what doesn’t look normal,” said Christie Lin, a senior scientist at the company.
Surgeons at UW Hospital are expected to be among the first to use the device, within the next two months, before a nationwide launch later this year, said Adam Uselmann, CEO.
The device is approved to image blood flow and the movement of fluid through tissue in vascular, gastrointestinal, organ transplant, reconstructive and micro surgeries.
OnLume has raised $1.8 million in grants and angel investment, and is raising a Series A round of financing for product development and commercialization, Uselmann said.
Founders include Uselmann, Thomas “Rock” Mackie, Benjamin Titz, Andreas Velten and Kevin Eliceiri.
Mackie co-founded TomoTherapy, an image-guided radiation treatment company in Madison that in 2011 was sold to Accuray, of Sunnyvale, California, for $277 million. Manufacturing continues in Madison.

Recent Comments